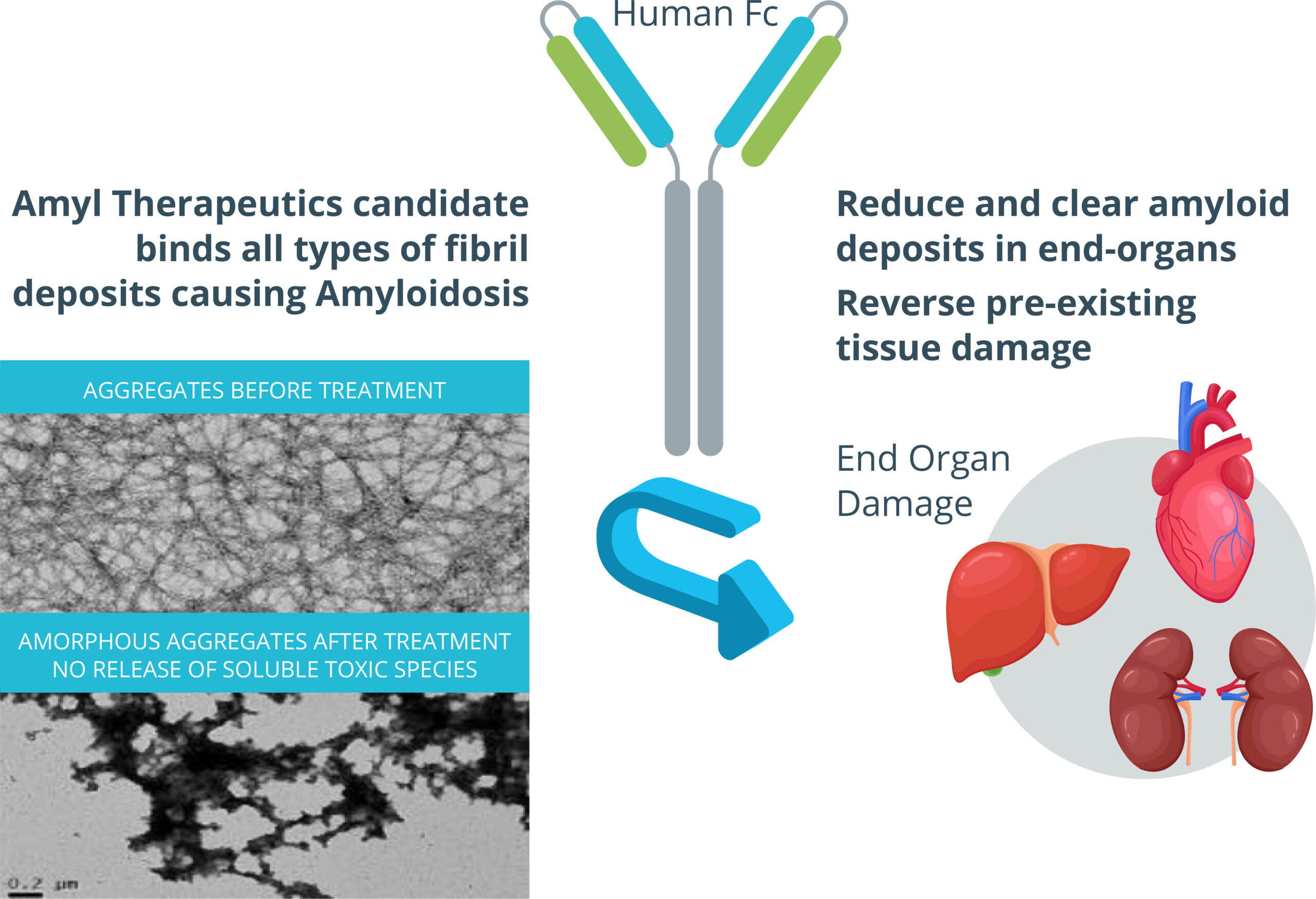
Our Science
Amyl Therapeutics novel approach to treat protein misfolding diseases is based on the fact that many toxic aggregates of misfolded proteins share a common characteristic – the amyloid protein fold – that represents a unique target for drug development.
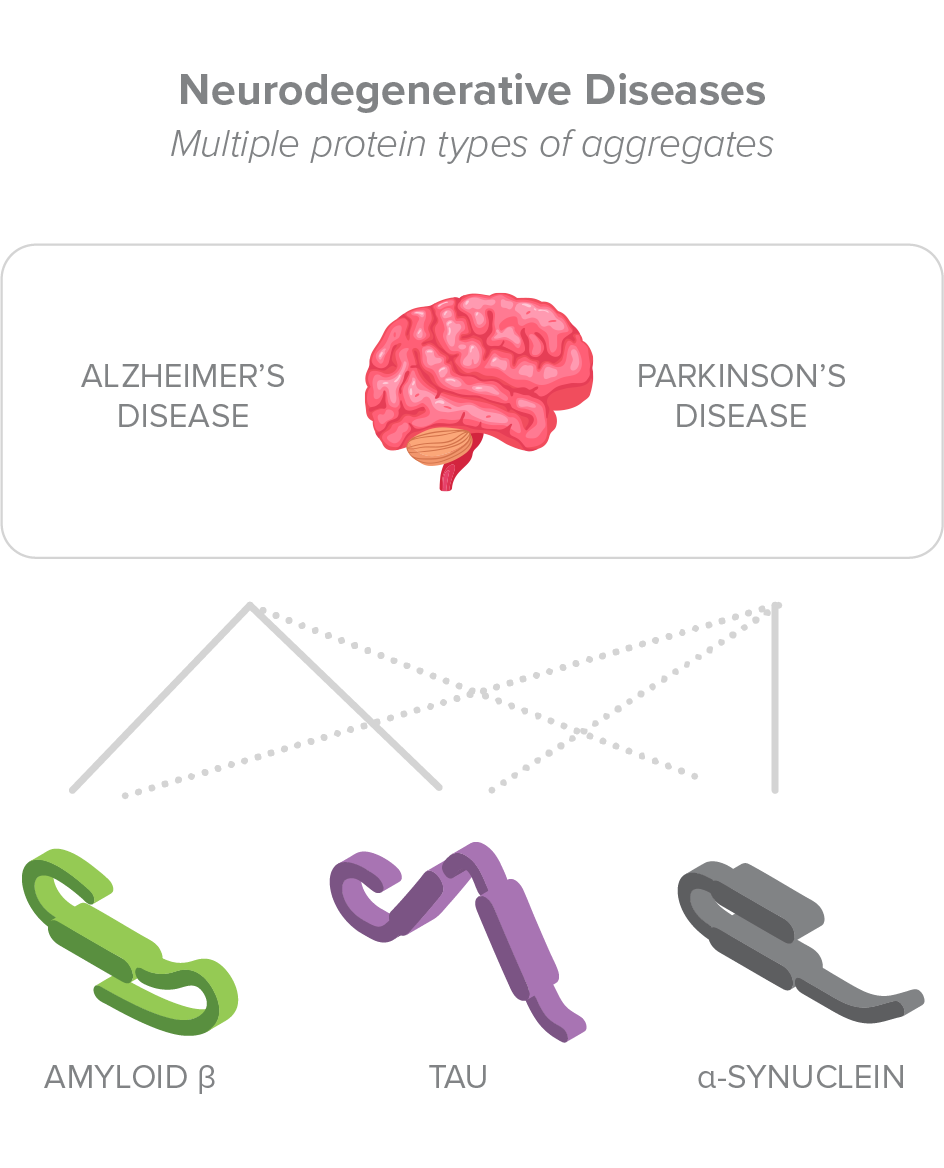
Most current therapies being investigated for diseases caused by deposits of amyloid fibrils target only a single type of misfolded protein, not addressing the fact that these diseases are often characterized by the buildup of multiple types of pathologic protein aggregates.
Amyl therapeutics novel proprietary technology, simultaneously targets multiple misfolded proteins implicated in both progressive peripheral diseases and neurodegenerative, potentially creating a more robust response that could be suitable for patients at all stages of disease.
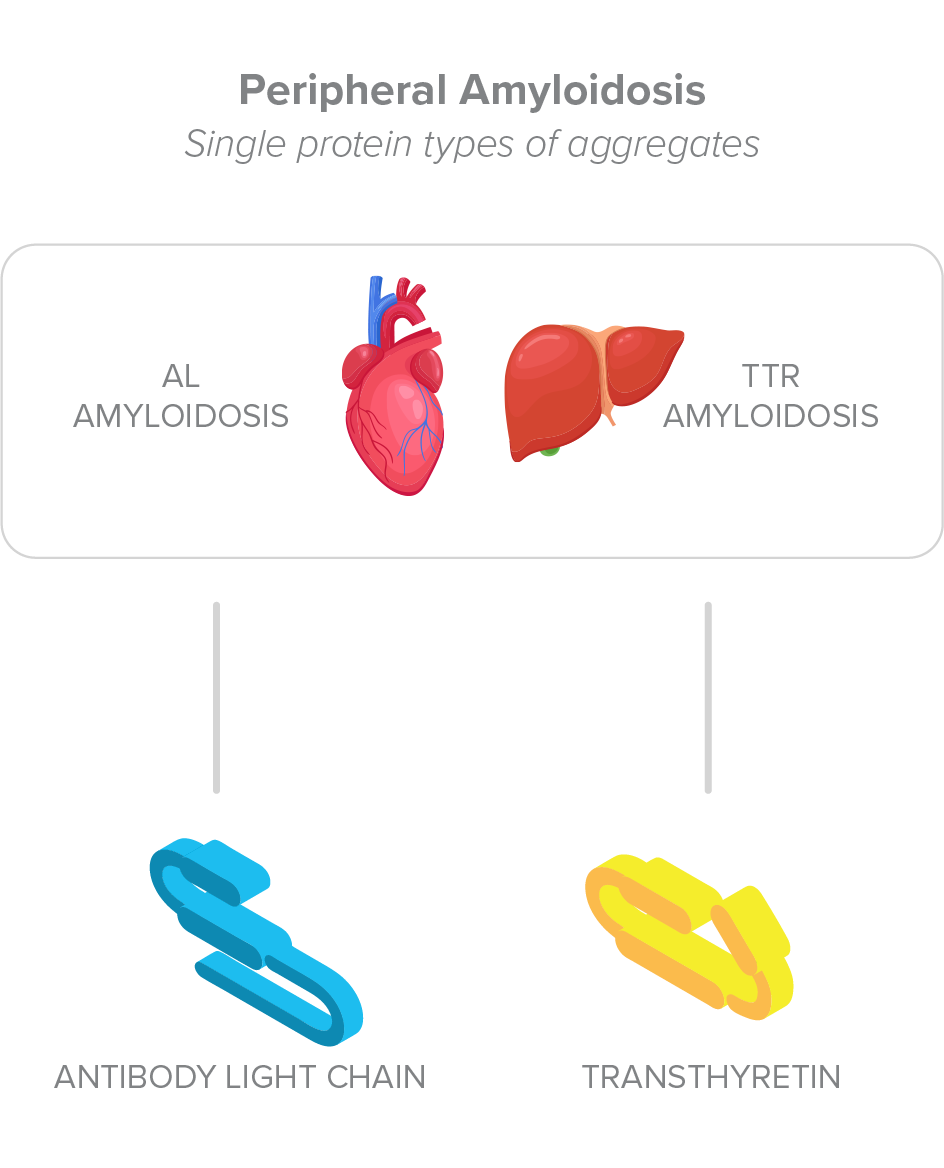
Amyl Therapeutics therapies use a novel mechanism to bind to the amyloid fold of toxic protein aggregates of various types, including antibody light chain (AL) or transthyretin (TTR) aggregates, which accumulate in peripheral organs to cause systemic amyloidosis diseases, but also amyloid-β (Aβ), Tau, and α-synuclein aggregates, which accumulate in the brain to cause Alzheimer’s and Parkinson’s disease.1,2
Our therapies are designed to prevent the further accumulation of aggregates and clear existing aggregates from the peripheral organs and the brain, while also blocking further cell-to-cell spread of misfolded proteins. We believe these therapies offer a breakthrough approach to treating protein misfolding diseases.
An urgent need
Proteins are large, exquisitely folded molecules that play essential and diverse roles in the human body. When normal protein folding is disrupted, these misfolded proteins clump together – binding to form toxic aggregates.
Protein misfolding is a characteristic of certain rare diseases caused by systemic accumulation of amyloid protein aggregates in peripheral organs such as the heart, the liver, kidneys and nerves. Examples of systemic amyloidosis diseases with accumulation of tissue-damaging aggregates include several types of transthyretin (TTR) amyloidosis and light chain (AL) amyloidosis.
There is a critical unmet need to develop novel treatments for protein misfolding diseases, for which no approved disease-modifying treatments currently exist.
Systemic amyloidosis diseases represent a broad collection of orphan diseases in which misfolded proteins can cause peripheral organ damages. Prominent among these diseases are transthyretin amyloidosis (ATTR), with the inherited versions of the disease affecting ~50,000 persons worldwide, and non-inherited ATTR affecting ~90% of persons >90 years old worldwide3-5; and antibody light chain (AL) amyloidosis with up to 4,000 new cases per year in the US. Both ATTR and AL amyloidosis can be fatal diseases that may be treatable using protein aggregate reduction therapy, such as Amyl’s candidate, to rescue organ function.
- Ando et al., Orphanet J Rare Dis, 2013 (Orphanet Journal of Rare Diseases)
- Ruberg et al., Circulation, 2012 (Circulation)
- Amyloidosis Research Consortium, Guidance for Industry – AL Amyloidosis – Developing Drugs for Treatment, Dec 2016 (www.arci.org)
- Krishnan R et al doi.org/10.1016/j.jmb.2014.04.015
- Asp E et al. doi.org/10.1016/j.jmb.2019.03.022